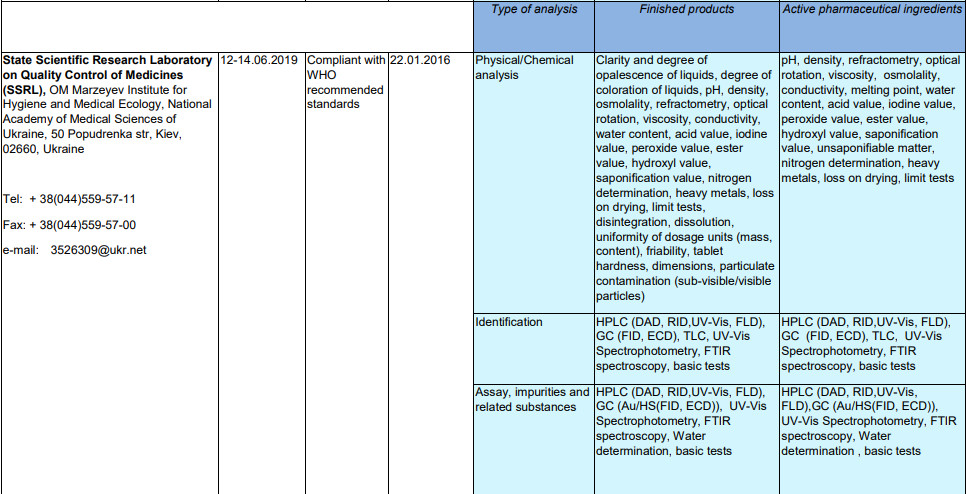
On January 22, 2016, the Laboratory received confirmation of WHO's pre-qualification, whose program was designed to provide the population with quality medicines.
Recognition of the compliance of the Laboratory with the requirements of international standards is a high estimation, confirmation of readiness of the laboratory to enter the path of international cooperation.
The laboratory is included in the List of pre-qualified WHO quality control laboratories considered acceptable for use by UN agencies and other international organizations.
The Laboratory confirms its compliance with the pre-qualification requirements by reporting to the WHO on its activities on quality control of medicinal products during the calendar year.